SAFEBREAK® VASCULAR
A BREAKAWAY DEVICE FOR ALL VASCULAR ACCESS LINES
SafeBreak Removes Harmful Forces From All IV Lines
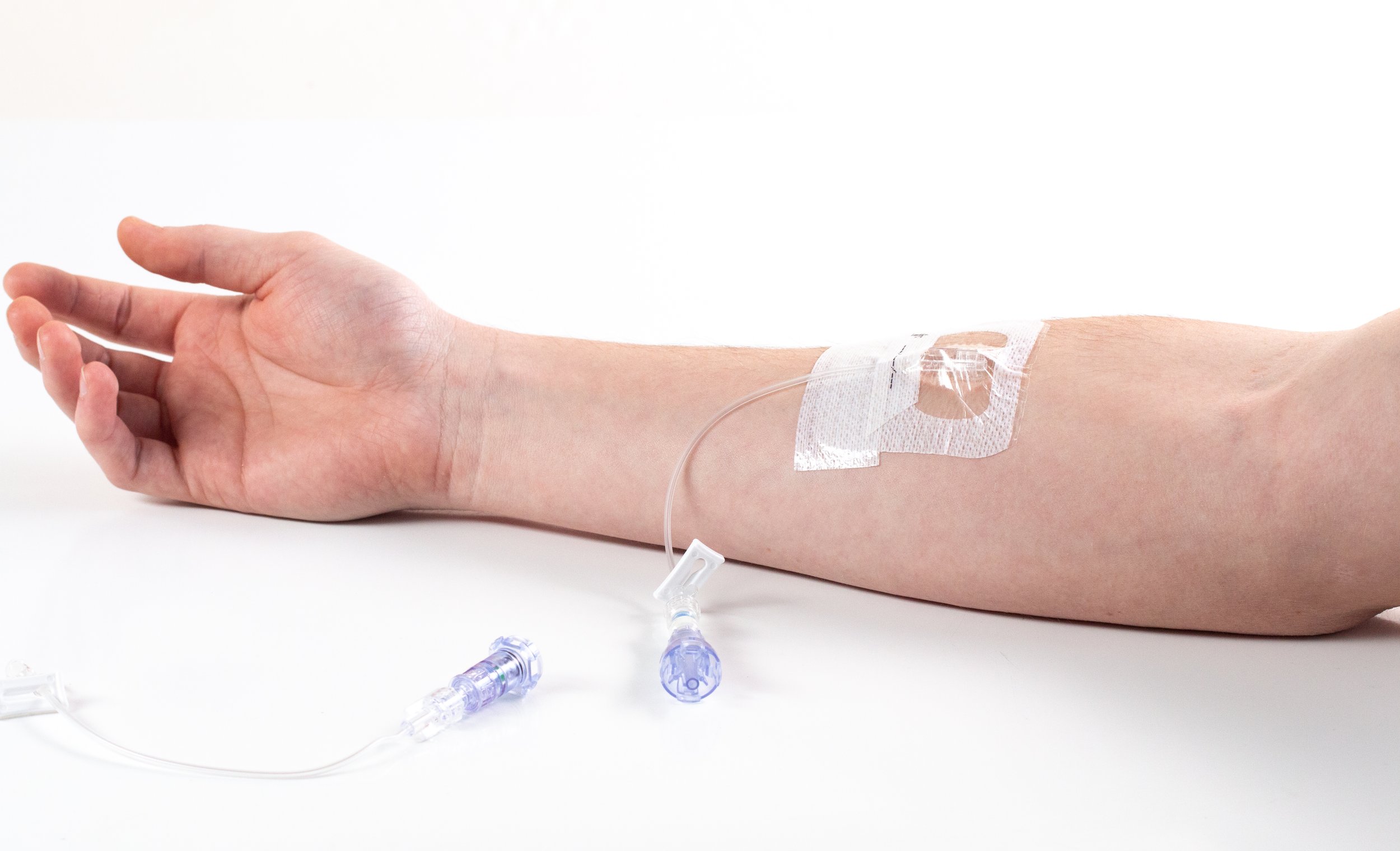
Harmful forces on IV lines can lead to dressing disruption, phlebitis, dislodgement, occlusion, infiltration and ultimately to an IV restart. The IV line shown in Figure 1 below has not dislodged, but no one should have a damaging force like this placed on their IV line. SafeBreak Vascular separates, as shown in the picture in Figure 2, to remove these harmful forces, protecting the patient and the IV catheter.
Figure 1. A 7 lb force applied to a peripheral IV line on a 50 year old male's arm.
Figure 2. SafeBreak Vascular separated
SafeBreak Works - Randomized Controlled Trial
In a 2021 randomized clinical trial conducted at Hartford Hospital in Hartford, CT with 287 patients, SafeBreak was shown to reduce overall IV complications requiring an IV restart by 44% and caused no additional delays in therapy for patients. Table 1 shows the results from the D.I.P.P.E.R. Study (Dislodgment, Infiltration, Phlebitis Prevention Eliminating PIV Restarts). Although the control group had a very low overall complication rate of 27%, the SafeBreak group experienced 44% less complications and had an overall complication rate of only 15%. (1) Click here for a copy of the D.I.P.P.E.R. Study White Paper.
Fast And Easy To Install
SafeBreak Vascular is inserted between the IV tubing from the pump and the catheter in the patient's arm. The medical professional first attaches SafeBreak to the IV tubing as seen in Figure 3. The device is then primed (completely filled with fluid), ensuring no air is left inside the device. Next, the luer connection on the needleless connector from the patient's IV access site is scrubbed, as seen in Figure 4. SafeBreak Vascular is then connected to the patient's needleless connector, as shown in Figure 5.
Figure 3. SafeBreak Vascular is connected to the line coming from the IV pump and primed
Figure 4. The needleless connector hub is then cleaned
Figure 5. SafeBreak Vascular is connected to the needleless connector and the infusion is started.
After SafeBreak Vascular is installed in the line, the IV infusion can begin. For full instructions on how to use SafeBreak Vascular, please consult the product's Instructions for Use.
Clinical Benefits Of Using SafeBreak Vascular
RESTART IV INFUSION QUICKLY WITHOUT A NEEDLESTICK
Restarting a IV normally requires finding a new vein and at least one additional needlestick. The average delay in therapy in one clinical study shows that the patient normally goes without IV medical treatment for 55 minutes.(2) In the D.I.P.P.E.R. study, the average patient requiring an IV restart experienced 86 minutes delay in therapy. SafeBreak Vascular can eliminate the tedious and stressful process of starting a new IV line. Both of these studies were performed at hospitals that use a Vascular Access Team to start all peripheral IVs. If SafeBreak is installed in an IV line and separates, the separated SafeBreak components can be thrown away and a new SafeBreak installed in only 5 minutes.(1) The patient’s IV infusion can be restarted with no mess to clean up, no drugs to reorder, and most importantly, without an additional needlestick.
Exposed valves and potential bacteria entry point
exposed valves
REDUCES BLOOD LOSS AND MEDICINE SPILLS
Once SafeBreak Vascular separates, a valve on each end of the device closes. The valve connected to the line that goes to the IV pump closes to stop the flow of medicine and causes the IV pump to alarm. The valve on the line connected to the patient closes and prevents the patient from bleeding. These valves are recessed, inhibiting the likelihood of contamination or infection. Currently when an IV dislodges, the IV pump continues to pump medication on the floor or in the patient's bed until someone turns the pump off.
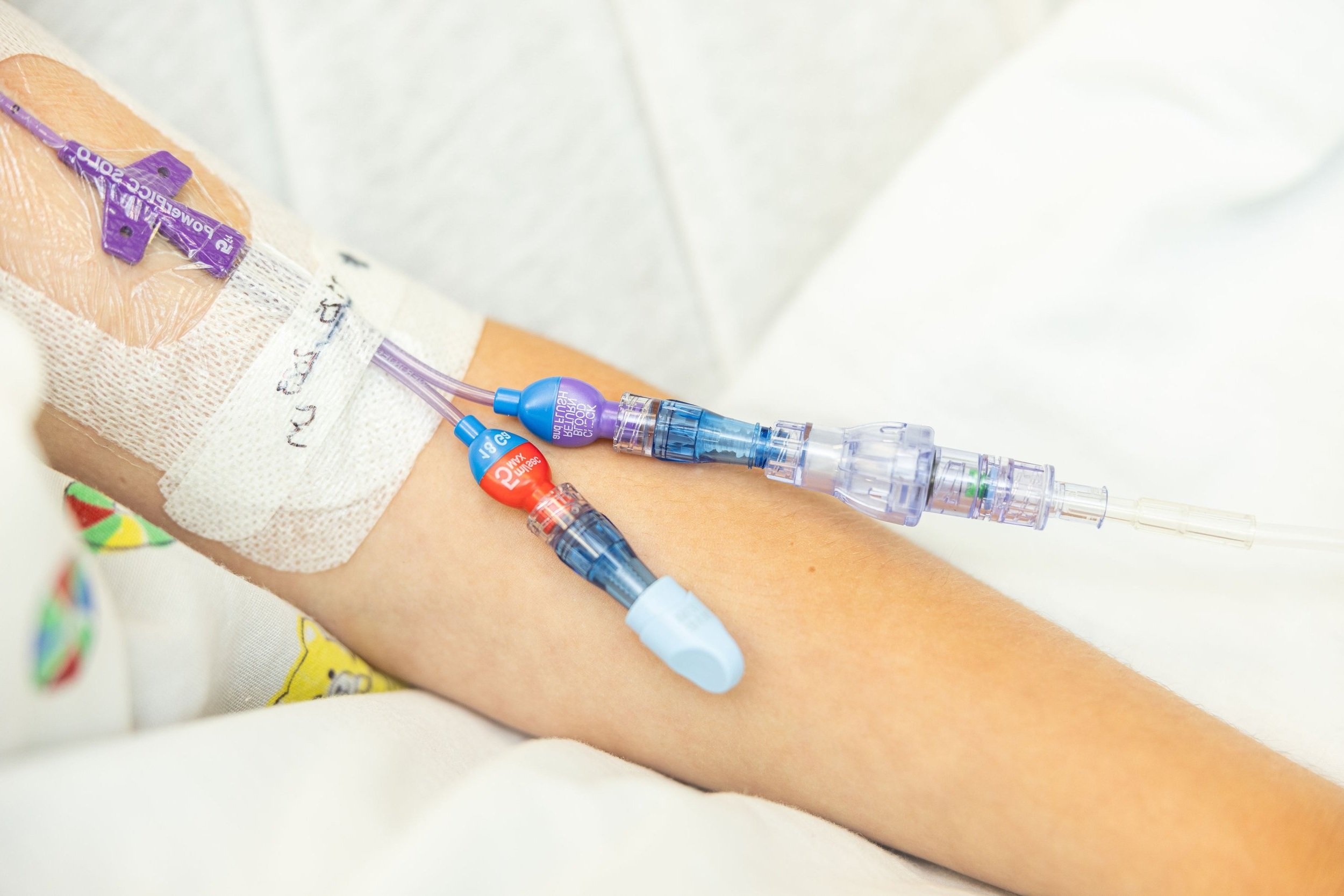
SafeBreak Vascular in the infusion line
Figure 6. SafeBreak IV installed in a peripheral IV line.
REASSEMBLY NOT POSSIBLE
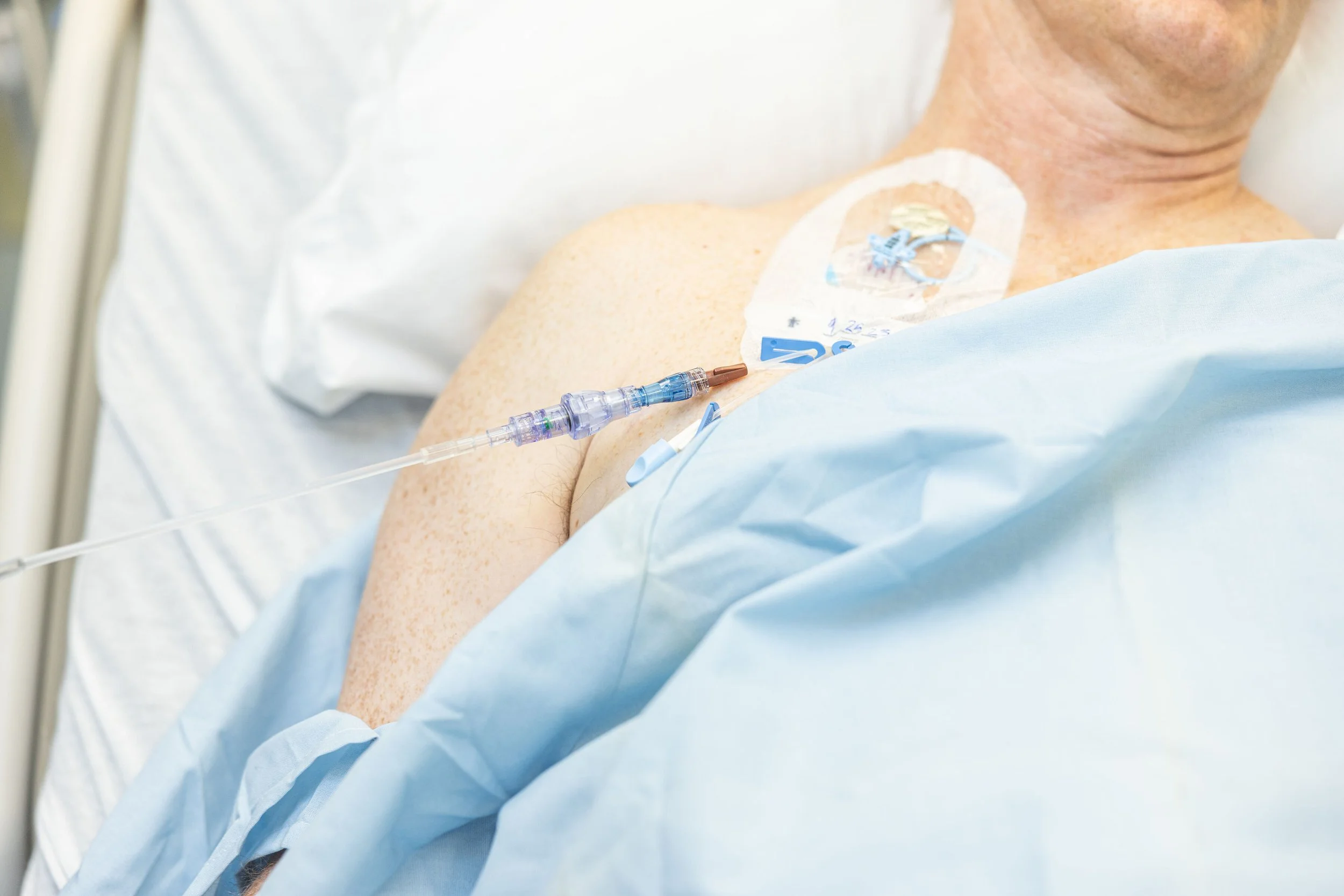
It is very important that a well intending family member or medical personnel are unable to put SafeBreak Vascular back together once it has separated, because the disconnected IV line might fall into the patient's hospital bed or onto the floor and become contaminated. If someone were to put the device back together, the infusion would run through a potentially contaminated line, which could lead to an infection. SafeBreak Vascular has a proprietary anti-reconnect feature and prevents anyone from accidentally putting the device back together. If SafeBreak is separated, the medical team has up to 2 hours to change out the device. After 2 hours, the entire IV must be restarted.
Figure 7. SafeBreak IV installed in a central line.
SafeBreak Vascular Separated
Competitive Device
1 Data on file.
2 Jenks, C., et al., The Ripple Effect - Analysis of the Cascade of Events Associated with Peripheral IV Loss and the Impact on Nurse Workflow and Resource Utilization. Association for Vascular Access Annual Scientific Meeting Poster Exhibit. 2018.
MKG-0012 Rev 05
THE ONLY BREAKAWAY DEVICE WITH INFECTION CONTROL TECHNOLOGY
SafeBreak Vascular was specifically designed with recessed valves to reduce the likelihood of potential infection. It is important that an IV line remains protected when SafeBreak separates. It simply does not make sense to protect the IV line from complications with a breakaway device and then leave the line susceptible to an infection.
Competitive devices have exposed valves that can easily come in contact with bacteria when the IV line separates. SafeBreak’s valves are buried inside the device so that bacterial contact is limited and so that a well meaning patient or nurse does not accidentally press on or manipulate the valves. Pressing on the valves in competitive devices can cause them to open and to leak fluids, blood or medication. This can not happen with SafeBreak.
Lineus Medical has conducted rigorous microbial ingress testing and has completed multiple veterinary and human clinical trials that show SafeBreak’s recessed valves keep the line clean for up to two hours when the IV line is separated (1).