CLINICAL EVIDENCE
D.I.P.P.E.R. STUDY - Randomized Controlled Trial(1)
Dislodgement, Infiltration, Phlebitis Prevention Eliminating Restarts
In 2021, Lineus completed a randomized controlled trial (RCT) at Hartford Hospital in Hartford, CT. This 287 patient study was focused on showing SafeBreak’s impact on infiltration, phlebitis and dislodgement.
The control group (N=144) in this study received the standard of care, while the experimental group (N=143) had SafeBreak Vascular placed on their peripheral IV line for the duration of their enrollment. Multiple Med/Surg units and ICU Step Down units were included in the study. All IV complications, SafeBreak separations, delays in therapy, and adverse events were tracked, as well as a host of other data points.
The primary endpoint of the study was to determine if the use of SafeBreak had an impact on PIV catheter mechanical complication rates (dislodgement, infiltration, and phlebitis) when compared to a control group receiving the standard of care. Lee Steere, RN, CRNI, VA-BC was the principal investigator of the study.
DIPPER Study Results
This randomized, controlled study demonstrated that the use of SafeBreak decreased the rate of overall mechanical complications requiring an IV restart and did not increase adverse events in patients receiving infusions via PIV catheter. Specifically, SafeBreak showed a combined statistically significant reduction in phlebitis, dislodgement, and infiltration. The control group had an IV complication rate of 27.1%, while the SafeBreak group had an IV complication rate of 15%. This represents a 44% reduction in overall IV complications. The randomized clinical study proved that the separation force of SafeBreak Vascular is effective in reducing the overall IV complication rates as well as reductions in individual complications when comparing the control group to the SafeBreak group.
DIPPER study results
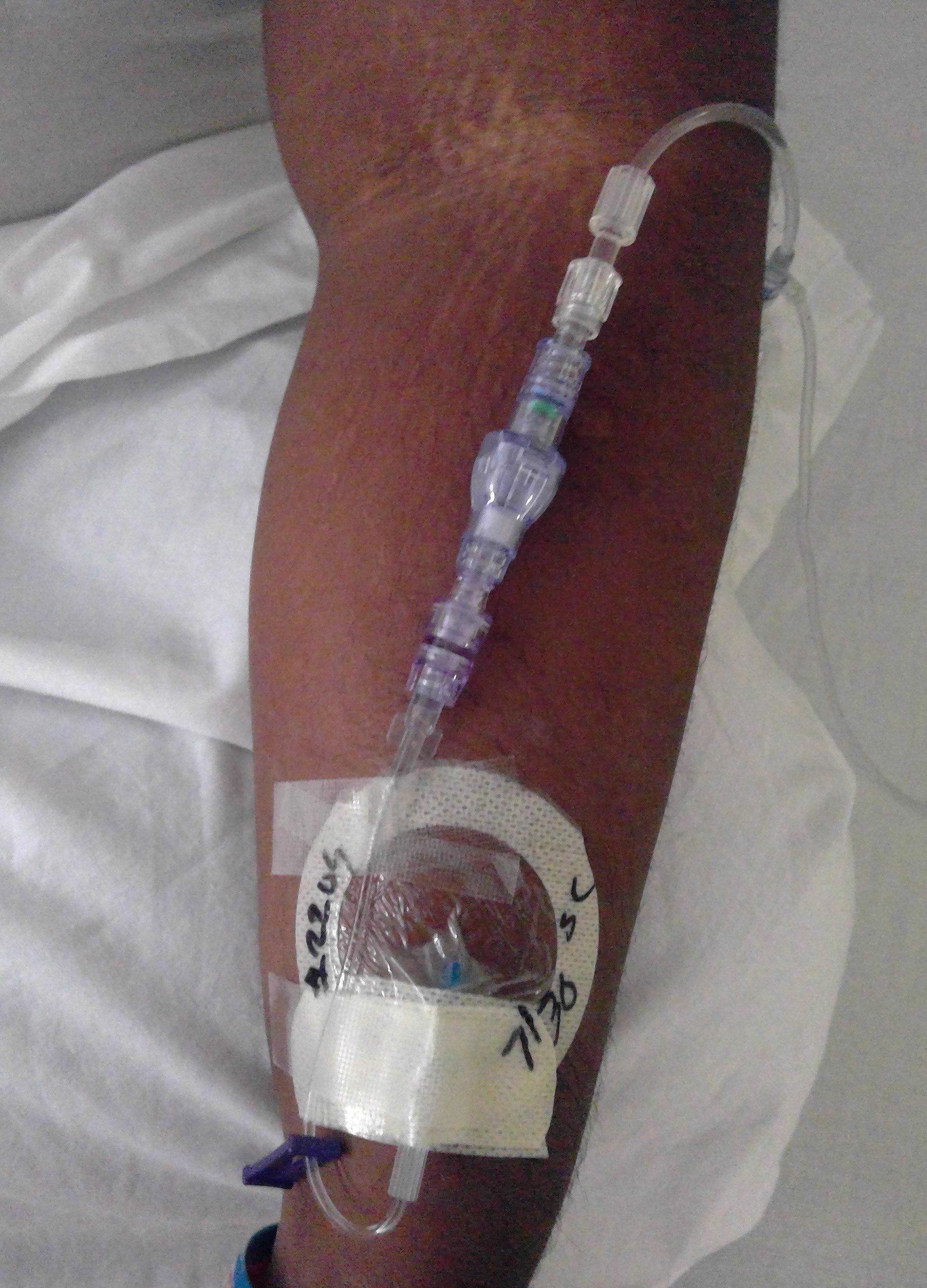
Patient in the control group without SafeBreak on their IV line
Patient in the expiermental group with SafeBreak on their IV line
Ultrasound Catheter Movement Study(2)
In 2023, Lineus completed a prospective blinded trial in an outpatient research laboratory. This study was focused on examining the change in movement (millimeters) of a PIVC by applying randomized external forces. The healthy volunteers (N=11) were given additional securement at PIVC insertion point and under the securement dressing to ensure the pull force’s impact was targeted as directly as possible to the PIVC insertion point and to help prevent dislodgement. Randomized pull forces of 4, 5, and 6 lbs were applied to the PIVC at a 20-deree angle to the subject’s arm.
Ultrasound Catheter Movement Study Results
Using ultrasound video to analyze the movement of the peripheral IV catheter, it was discovered that the catheter made contact with the vein wall and remained in contact with the vein wall after the pull force was released 54.6% of the time. Additionally, the random pull forces of 4, 5, and 6lbs resulted in a range of catheter movement from 1.30 to 8.10mm. Dr. Amit Bahl was the principal investigator of the study.
Pull force test device at 20-degree angle
Ultrasound video of vein interior when force is applied to PIVC
Microbial Ingress Study(1)
This study is a review of bench-top and clinical studies demonstrating effectiveness of recessed valves in SafeBreak Vascular in preventing microbial ingress. The purpose of recessed valves is two-fold. The first objective is to eliminate both wasted medication and to prevent the clean ups that come from spilled medication and blood. Secondly, the recessed valves provide a barrier to inhibit bacteria from passing into the IV line and potentially causing infection. The results of the microbial ingress testing demonstrated that SafeBreak Vascular acts as an effective barrier to multiple, microbial contamination events.
IV Administration Tubing Side Inoculation Method
IV Patient Tubing Side Inoculation Method
Barnes- Jewish Study (1)
In 2018 this prospective, non-randomized study, the study group had an overall IV complication rate of 26.2% compared to prospective randomized peripheral IV catheter studies in clinical literature of 46%. The study group had lower complications rates for every mechanical complication, though this study was not powered to prove a statistically significant reduction in these mechanical complications. This study included the use of SafeBreak on all types of IVs placed in the patient: peripheral, PICC and Central lines. The principal investigator for this study was Dr. Grant Bochicchio.
Ripple Effect Study(3)
In 2018 an analysis of the cascade of events associated with peripheral IV loss due to IV dislodgement and the impact on nurse workflow and resource utilization was conducted. The primary objective of this study was to characterize and evaluate all the costs of an IV dislodgement in a general medical hospital. A questionnaire was utilized to determine patient demographics, causes of dislodgement, disruption of care, expense of dislodgement cleanup, and the expense of establishing a replacement IV for 51 sequential patients experiencing IV dislodgement over a three- week period.
Results of Ripple Effect Study
The average cost of a PIVC dislodgement was $47.74 with 77.5% accounting for supplies and medication lost and 22.5% associated with the labor required to start a new IV. The study showed that 50.9% of cases happened in critical care with the cause of dislodgement being 44% due to a cognitive issue. The average time associated with the patient experience a delay in therapy while an IV was restarted was 55 minutes with the longest time reported being 4 hours. The nursing labor associated with a nurse restarting an IV was 8 minutes with an average of 1.3 sticks per patient. The principal investigator was Dr. Mark Graves.
*IV access performed by a vascular access team likely providing first stick insertion results that are better than average.
Securement Pull Force Study(4)
In 2018 a performance test of peripheral IV securement (Tegaderm I.V. Advanced (1683), SorbaView Shield PIV, Grip-Lok, and StatLock) closely analyzed how tension on an IV line impacts catheter function by understanding the nature of infusion component failure during simulated pull force scenarios. Despite dressing technology’s advancement over the past decade with door, wings, and overlapping components, mechanical IV complications (dislodgement, infiltration, occlusion, and phlebitis) can still arise. The study was conducted using pull forces from a 90-degree axial, a 0-degree axial, and a 90-degree shear pull force angle.
Results of Securement Pull Force Study
The performance characteristics of the studied securement devices varied greatly. Forces below 4.3 lbs were generally safe for the catheter and did not cause failure mode to occur. Forces greater than 4.3 lbs frequently caused dressing disruption and catheter hub movement. The full dressing dislodgement had a 17.9% occurrence, partial dressing disruption had a 52.4% occurrence and no dressing disruption had a 29.8% occurrence. The graph below displays the mean force at loss of catheter function for each dressing type and what the catheter status was at the mean force.
90-degree Axial Pull Force from study
MKG-0078 REV 03
Data on File
Bahl A, Clement V, DiLoreto E, Mielke N, Carr A, Panza G, Gibson SM. Evaluating the impact of external forces on peripheral intravenous catheter movement using ultrasound: A randomized pilot study. J Vasc Access. 2024 Jan 5:11297298231222052. doi: 10.1177/11297298231222052. Epub ahead of print. PMID: 38183179.
Jenks, C., et al., The Ripple Effect – Analysis of the Cascade of Events Associated with Peripheral IV Loss and the Impact on Nurse Workflow and Resource Utilization. Association for Vascular Access Annual Scientific Meeting 2018
Jones, S., et al., Performance Testing of Peripheral IV Securement in a Clinically Simulated Environment. World Congress on Vascular Access Scientific Meeting 2017